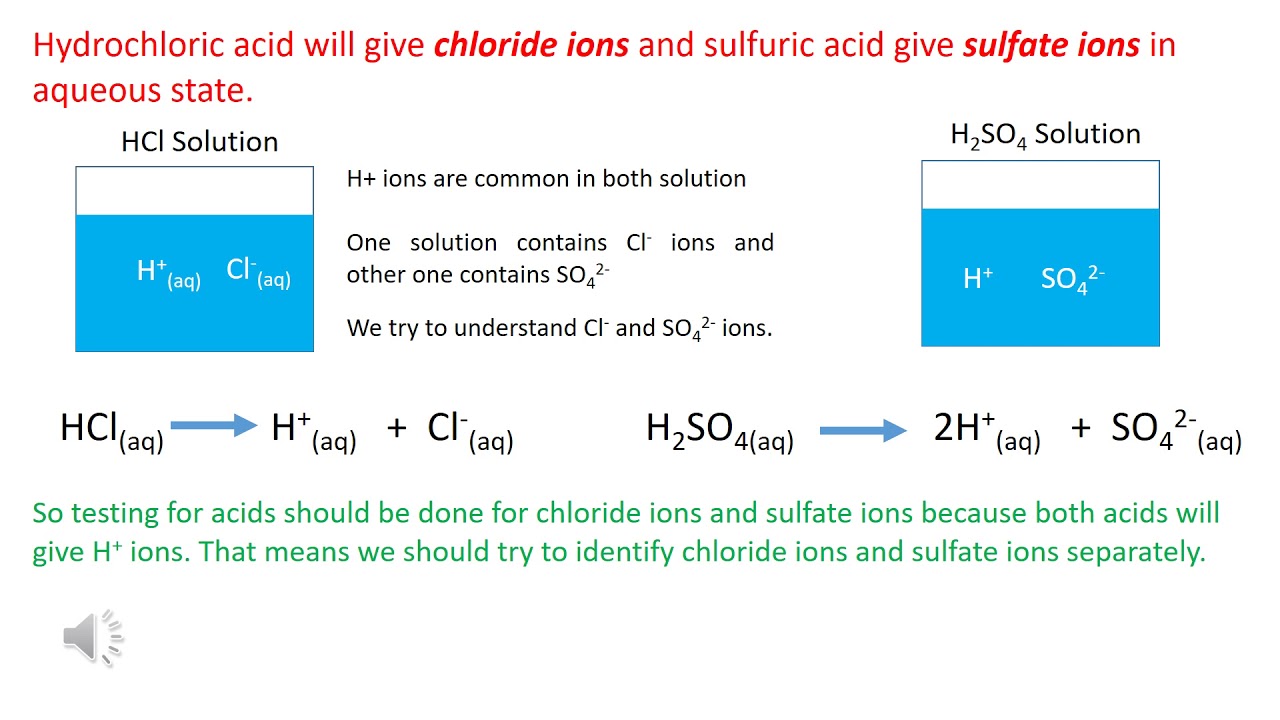
Dealing with hazardous waste is a serious business, and hydrochloric acid (HCl) waste is no exception. Improper HCl waste management can lead to environmental damage, hefty fines, and even endanger human health. This guide provides a comprehensive overview of hydrochloric acid waste disposal, offering practical strategies for safe and compliant handling.
Hydrochloric acid, a powerful corrosive substance, is widely used across various industries, from steel pickling and oil well acidizing to laboratory research and swimming pool maintenance. The widespread application of this powerful chemical inevitably leads to the generation of HCl waste, demanding careful management and disposal strategies.
The history of hydrochloric acid use stretches back centuries, but modern industrial applications have significantly increased the volume of waste generated. Early regulations surrounding HCl disposal were often lax, leading to environmental contamination. Today, however, strict guidelines and best practices ensure responsible handling of this hazardous waste stream.
The importance of proper hydrochloric acid waste disposal cannot be overstated. Environmental protection is paramount. Improper disposal can contaminate soil and waterways, harming ecosystems and potentially impacting human health through the food chain. Furthermore, compliance with regulations is crucial to avoid penalties and legal repercussions. Responsible HCl waste management is not just good practice, it's a necessity.
One of the primary issues associated with HCl waste disposal is its corrosive nature. Direct disposal without proper treatment can damage piping and infrastructure. Neutralization is a key step in managing HCl waste, typically involving reacting the acid with a base like sodium hydroxide or calcium hydroxide to form a less hazardous salt and water.
Hydrochloric acid waste neutralization is the process of reducing the acidity of the waste to a safe pH level, typically around neutral (pH 7). This involves carefully adding a base to the acid while monitoring the pH. Simple examples include adding lime slurry to neutralize larger volumes of HCl waste or using sodium bicarbonate for smaller spills.
Benefits of Proper Hydrochloric Acid Waste Disposal:
1. Environmental Protection: Proper HCl neutralization and disposal safeguards ecosystems by preventing contamination of soil and water resources, thus preserving biodiversity.
2. Regulatory Compliance: Adhering to stringent HCl waste management guidelines ensures compliance with environmental regulations, avoiding hefty penalties and legal issues.
3. Safety Enhancement: Safe HCl disposal practices protect workers and the public from exposure to this corrosive substance, minimizing risks of chemical burns and other health hazards.
Action Plan for HCl Waste Disposal:
1. Characterize Waste: Determine the concentration and volume of the HCl waste.
2. Choose Neutralization Method: Select an appropriate base and neutralization procedure.
3. Implement Neutralization: Carefully add the base while monitoring pH.
4. Dispose of Neutralized Waste: Follow local regulations for disposal of the neutralized solution.
Best Practices for Hydrochloric Acid Waste Disposal:
1. Always wear appropriate personal protective equipment (PPE).
2. Store HCl waste in designated, chemically compatible containers.
3. Neutralize waste in a well-ventilated area.
4. Regularly inspect storage and handling equipment.
5. Maintain detailed records of waste generation and disposal.
Challenges and Solutions:
1. High Concentrations: Highly concentrated HCl requires careful dilution before neutralization. Solution: Slowly add water to the acid while stirring continuously.
2. Exothermic Reaction: Neutralization generates heat, which can be hazardous. Solution: Control the rate of base addition to manage temperature.
FAQs:
1. What is the pH of neutralized HCl waste? Ideally, it should be near neutral (pH 7).
2. Can I pour HCl down the drain? No, it's illegal and environmentally damaging.
3. What are the risks of improper HCl disposal? Environmental contamination, fines, and health hazards.
4. What is the best way to neutralize HCl? By carefully reacting it with a suitable base.
5. What PPE should I wear when handling HCl waste? Gloves, goggles, lab coat, and closed-toe shoes.
6. What are the regulations regarding HCl disposal? These vary by location, consult local authorities.
7. How should I store HCl waste? In designated, chemically compatible containers.
8. What should I do in case of an HCl spill? Contain the spill and neutralize it with a suitable base.
Tips and Tricks: Always add acid to water, never the reverse. Ensure adequate ventilation during neutralization. Regularly inspect and maintain equipment.
In conclusion, hydrochloric acid waste disposal is a crucial aspect of responsible chemical management. From protecting the environment to ensuring worker safety and complying with regulations, proper HCl waste handling offers numerous benefits. By understanding the neutralization process, following best practices, and addressing potential challenges, industries and individuals can contribute to a safer and more sustainable future. Proactive waste management not only mitigates risks but also demonstrates a commitment to environmental stewardship. Don't just dispose – manage your HCl waste responsibly for the benefit of all. Contact your local environmental agency for specific guidance on regulations and approved disposal methods in your area. Prioritize safety and environmental responsibility in all your HCl waste management practices.
Unlocking the magic of valspars top paint hues
Dodgeball domination corvallis oregons epic dodgeball scene
Elevating your waterfront sanctuary exploring boat lift investments