
Ever wondered how chemical reactions happen? What drives them forward, and why some release energy while others require it? The answer lies within the fascinating world of chemical potential energy diagrams. These visual tools provide a powerful lens into the energetic landscape of chemical transformations, allowing us to understand the intricate dance of molecules as they break and form bonds.
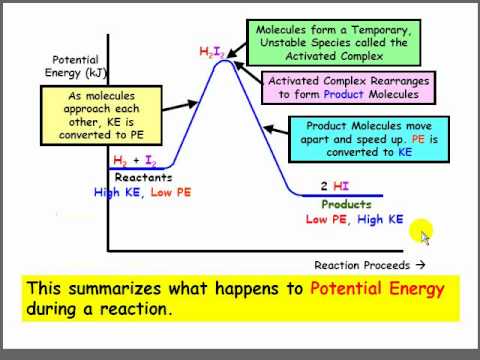
Chemical potential energy diagrams, also known as reaction coordinate diagrams, graphically represent the energy changes that occur throughout a chemical reaction. Imagine a rollercoaster – the climb represents the energy input needed to start the reaction, and the descent shows the energy released as the reaction progresses. These diagrams illustrate the energy pathway from reactants to products, providing key insights into the reaction's mechanism and feasibility.
The concept of a reaction's energy profile can be traced back to the late 19th and early 20th centuries as scientists began to understand the relationship between energy and chemical change. The development of thermodynamics and chemical kinetics paved the way for visually representing these energy changes, leading to the modern chemical potential energy diagram. These diagrams are indispensable tools for chemists, helping them predict reaction rates, understand the role of catalysts, and design more efficient chemical processes.
A central concept in understanding these diagrams is the activation energy – the energy barrier that reactants must overcome to transform into products. Think of it as the initial push needed to get the rollercoaster over the first hill. The higher the activation energy, the slower the reaction. Another important feature is the difference in energy between reactants and products, which determines whether a reaction is exothermic (releases energy) or endothermic (absorbs energy).
Understanding how to interpret these diagrams is crucial for anyone studying chemistry. By analyzing the peaks, valleys, and slopes of the energy profile, we can glean valuable information about the reaction's progress. For instance, a high activation energy suggests the reaction will proceed slowly, while a large difference in energy between reactants and products indicates a strong driving force for the reaction. This information is fundamental for designing and optimizing chemical reactions in various fields, from industrial chemistry to drug development.
One benefit of using chemical potential energy diagrams is predicting reaction rates. By visualizing the activation energy, we can estimate how fast a reaction will occur. A lower activation energy indicates a faster reaction rate. For example, the combustion of methane has a relatively low activation energy, resulting in a rapid reaction, while the rusting of iron has a high activation energy, explaining why it is a slow process.
Another advantage is understanding the role of catalysts. Catalysts are substances that speed up chemical reactions without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. Chemical potential energy diagrams can clearly illustrate how catalysts reduce the energy barrier, making the reaction proceed faster. For instance, enzymes are biological catalysts that accelerate biochemical reactions in our bodies.
These diagrams also help in designing and optimizing chemical processes. By understanding the energy changes involved, chemists can manipulate reaction conditions to favor desired outcomes. For example, increasing temperature often lowers the activation energy, increasing the reaction rate. This knowledge is applied in countless industrial processes, from synthesizing new materials to producing pharmaceuticals.
Advantages and Disadvantages of Chemical Potential Energy Diagrams
| Advantages | Disadvantages |
|---|---|
| Visual representation of energy changes | Simplified representation of complex reactions |
| Predicts reaction rates | Doesn't provide detailed mechanistic information |
| Illustrates the role of catalysts | Can be challenging to interpret for complex reactions |
One real-world example is the Haber-Bosch process, which is used to produce ammonia. Chemical potential energy diagrams are essential for understanding how the reaction conditions, such as temperature and pressure, affect the reaction rate and yield of ammonia.
Frequently Asked Question: What does a peak in a chemical potential energy diagram represent?
Answer: The peak represents the transition state, a high-energy intermediate state between reactants and products.
In conclusion, chemical potential energy diagrams are essential tools for understanding chemical reactions. They provide a visual representation of energy changes, allowing us to predict reaction rates, understand the role of catalysts, and design more efficient chemical processes. From understanding basic chemical principles to designing complex industrial processes, these diagrams are invaluable for anyone working with chemical transformations. By delving into the energetic landscape of reactions, we can unlock the secrets of chemical reactivity and pave the way for advancements in various scientific fields. So, the next time you encounter a chemical reaction, remember the power of the chemical potential energy diagram – a window into the dynamic world of molecular transformations.
The epic saga of super bowl results
Elevate your game finding the perfect bowling ball pro shop
Decoding song lyrics unraveling the mystery of tell me ma





:max_bytes(150000):strip_icc()/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



